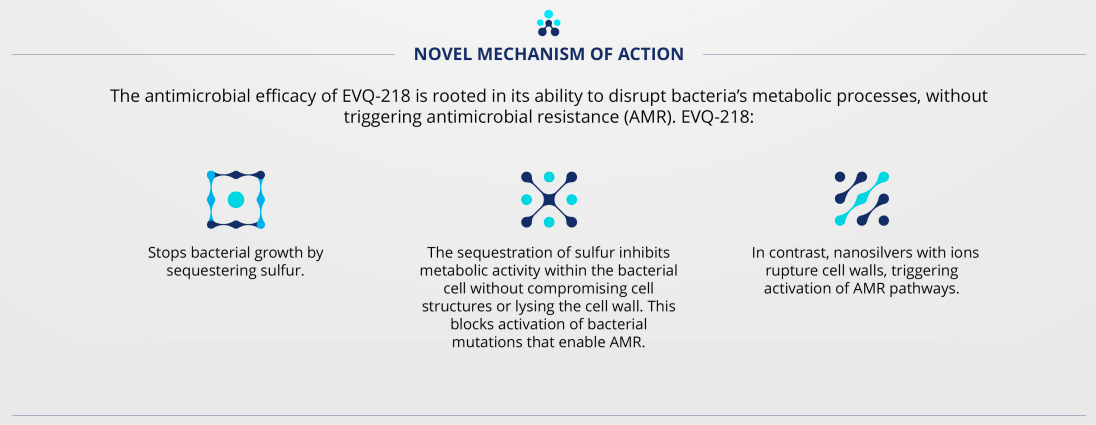
without creating antimicrobial resistance.

Admiral Brett P. Giroir
Former Assistant U.S. Secretary of Health
EVŌQ Nano is a nanoscience company that creates uniform, stable, sub-10 nm nanoparticles for a wide range of applications.
Our patented nanofabrication process requires only laser energy, ultrapure water, and pure silver metal. Ablation via a multiple, cross-laser system occurs at temperatures and pressures akin to diamond formation. The single-step, high-volume laser process generates stable, pure metal nanoparticles directly into pharmaceutical-grade water.
Shaun Rothwell, Chairman & CEO
David Parkinson, Chief Legal Officer & Board Member
Jeff Bennion, Chief Operations Officer
David Nilson, Chief Development Officer
William Niedermeyer, Founder & Chief Technology Officer
Kami Ball, VP of Accounting
Steve Savage, President of FUZE Technologies
Andrew Peterson, Chief Technical Officer
Charles Wright, President of Medtech & Bio
Nate Kerr, Vice President of Medical Operations
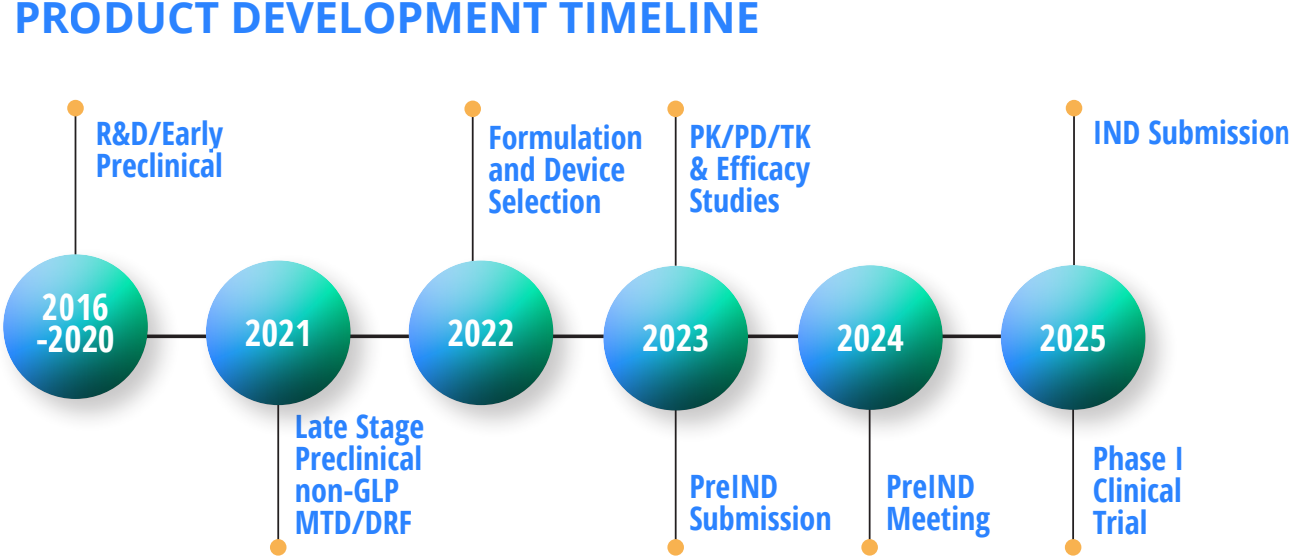
EVŌQ Nano is backed by $20 million in seed funding. The company has several R&D partnerships.
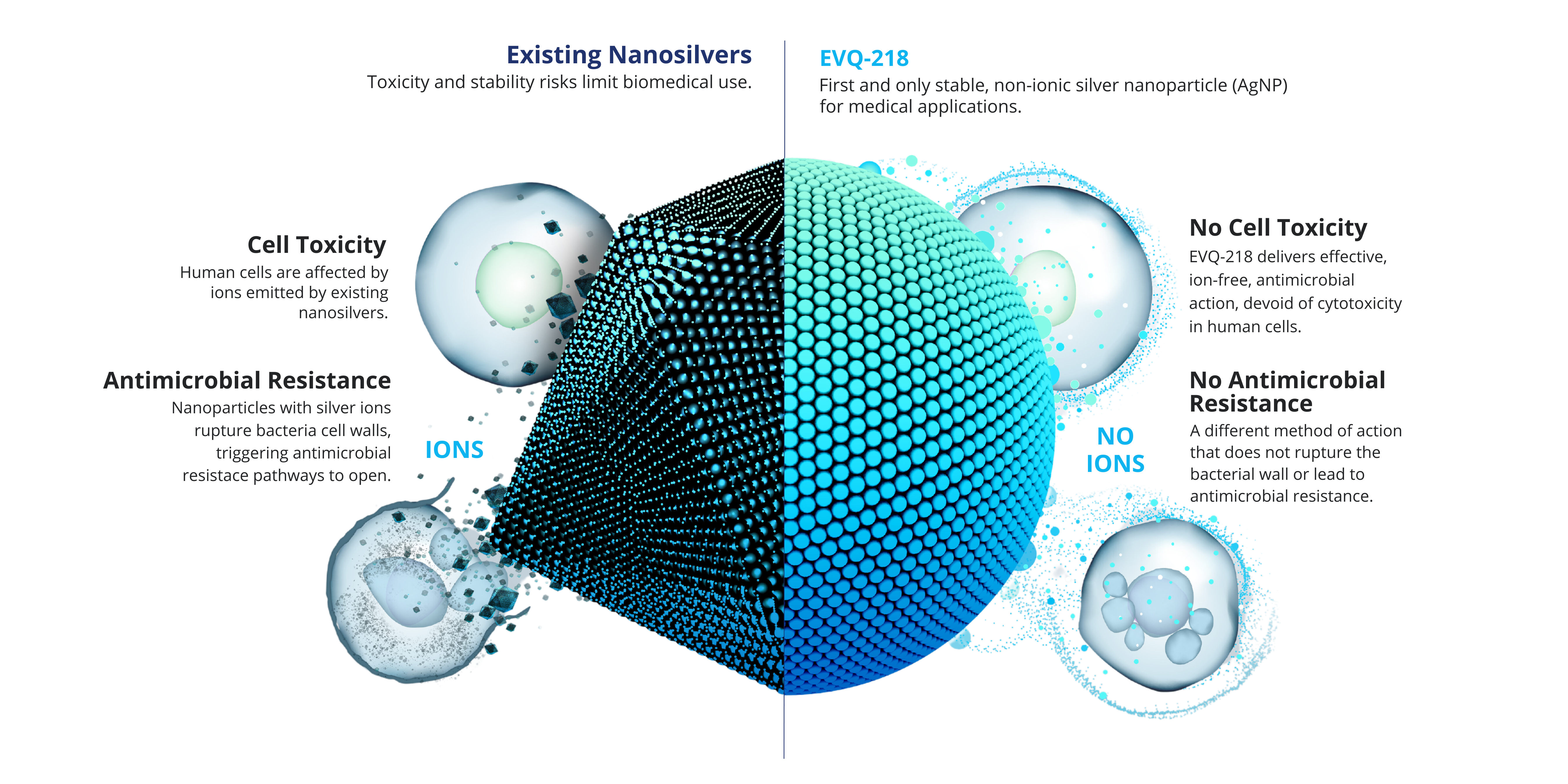
While silver’s antibacterial qualities have been known for centuries, it is widely established that the antimicrobial activity is due to ion emission, which poses toxicity risks for biomedical and consumer product applications.1-4
EVQ-218 is differentiated by dissolution and surface chemistry as the first stable, nonemissive, pure silver nanoparticle.5



The first therapeutic in development using EVQ-218 is for the treatment of pulmonary bacterial infections in patients with cystic fibrosis. Supported by 2 grants from the Cystic Fibrosis Foundation, research found an inhaled therapeutic using EVQ-218 demonstrated efficacy against pathogens linked to pulmonary infections:

EVŌQ Bio’s novel platform has the potential to enable therapeutic development for a broad spectrum of diseases.
DISEASE INDICATION
TARGET
R&D
IND ENABLING
Pulmonary Infection
(Cystic Fibrosis)
Pseudomonas aeruginosa Burkholderia cepacia Stenotrophomonas
Pulmonary Infection
(Bacterial Pneumonia)
Streptococcus pneumoiae
Streptococcus aureus
Streptococcus pyogenes
Klebsiella pneumoniae
Haemophilus influenzae
Pulmonary Infection
(Fungal)
Candida albicans
Aspergillus
Tuberculosis
Mycobacterium
tuberculosis
Cellulitis
Group A ß-hemolytic
streptococcus
Streptococcus pneumoiae
Staph MRSA
(Skin)
Methicillin-resistant
Staphylococcus aureus
Diabetic Foot Ulcer
Staphylococcus aureus
Streptococcus aureus
Pseudomonas aeruginosa
Seasonal Flu
Influenza A
COVID-19
SARS-CoV2


EVOQ Nano’s antimicrobial nanoplatform is well positioned to target the 1 million healthcare-associated infections (HAIs) caused by indwelling urinary catheters annually in the U.S.11 Bloodstream, pulmonary, and urinary tract infections are a significant cause of morbidity and increased mortality globally.12


Media Contact
Capwell Communications
[email protected]


FUZE Technologies is an antimicrobial solutions company serving the textile, hospitality, fitness, and workspace industries. Its EPA-approved antimicrobial technology is free of chemicals, toxicity, and environmental waste. The company has secured contracts with some of the world’s most notable brands, including Nike, Adidas, the New England Patriots, Marriott, and Hilton.
Please provide your information to discuss partnership or employment opportunities.


A lifelong scientist with a background in applied physics, EVŌQ Nano Co-Founder and Chief Technology Officer William Niedermeyer values focused research. He has led several research and development programs within instrumental ion physics in the private and corporate sectors, applying his physics background in several groundbreaking technologies. When the events of 9/11 suspended his doctoral studies in high-energy physics at the University of Utah, Niedermeyer pivoted to the burgeoning nanotechnology field, intrigued by its potential applications in medicine, renewable energy, and microelectronics.
With proceeds from his first company, in 2001 Niedermeyer started a small laboratory to investigate nanoparticles and their properties. Early efforts were frustrated, however, by recurring problems with nanoparticle impurity, uneven size distribution, agglomeration into large clumps, low energy emission, and colloidal instability. Plus, purchasing diverse nanoparticles in bulk to conduct research was incredibly expensive.
Motivated to remedy these limitations, Niedermeyer engineered his own nanofabrication process using modified laser equipment and parts from the previous Star Wars program at Lawrence Livermore National Laboratory. To his amazement, this invention produced nanoparticles unlike any he had studied. They were uniformly sized, surfactant-free, with consistent spherical morphology, and no ion emissions under standard and stressed conditions – properties that represented a significant advancement in nanoscience with the potential to enable new applications.
In 2011, Niedermeyer and his team patented the unique laser-based nanofabrication process and the resulting “Niedermeyer Particle.” Starting with 3 initial patents, the company has grown its intellectual portfolio to include 39 issued and 39 pending patents as of 2023.








































EVŌQ Nano is backed by $20 million in seed funding. The company has R&D partnerships with the University of Utah Nanofab & Health Core Sciences, National Science Foundation Materials Research Science and Engineering Center, Cystic Fibrosis Foundation, Charles River Laboratories, Lovelace Biomedical, Seattle Children’s Research Institute, Accugen Laboratories, Stillmeadow Inc, Healthcare Innovation & Technology Lab, Galbraith Laboratories, Nelson Laboratories, Emery Laboratories, University of Washington, Arizona State University – EELS, and Utah State University.

1. Stabryla, L. M.; Johnston, K. A.; Millstone, J. E.; Gilbertson, L.M. Emerging investigator series: it’s not all about the ion: support for particle-specific contributions to silver nanoparticle antimicrobial activity Environ. Sci. Nano 2018, 5, 2047−2068.
2. Kedziora, A.; Speruda, M.; Krzyzewska, E.; Rybka, J.; Bugla-Ploskonska, G. Similarities and Differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444.
3. Slavin, Y. N.; Asnis, J.; H.feli, U. O.; Bach, H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65.
4. Liao, C.; Li, Y.; Tjong, S. C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449.
5. Kennon BS, Niedermeyer WH. EVQ-218: Characterization of high-energy nanoparticles that measure up to NIST standards. ACS Omega. 2024. doi: 10.1021/acsomega.3c07745.
6. Cakic M., Glisic S., Nikolic G. Synthesis, characterization and antimicrobial activity of dextran sulphate stabilized silver nanoparticles. J. Mol. Struct. 2016;1110:156–161. doi: 10.1016/j.molstruc.2016.01.040
7. Yan X., He B., Liu L., Qu G., Shi J., Hu L., Jiang G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics. 2018;10:557–564. doi: 10.1039/C7MT00328E
8. Gamboa S.M., Rojas E.R., Martínez V.V., Vega-Baudrit J. Synthesis and characterization of silver nanoparticles and their application as an antibacterial agent. Int. J. Biosen. Bioelectron
9. Rajeshkumar S., Bharath L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem. Biol. Interact. 2017;273:219–227. doi: 10.1016/j.cbi.2017.06.019
10 Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J Funct Biomater. 2020 Dec; 11(4): 84. Published online 2020 Nov 26. doi: 10.3390/jfb11040084
11. Werneburg GT. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res Rep Urol. 2022 Apr 4;14:109-133. doi: 10.2147/RRU.S273663. PMID: 35402319; PMCID: PMC8992741.
12. Al-Rawajfah OM, Hewitt JB, Stetzer F, Cheema J. Length of stay and charges associated with health care acquired bloodstream infections. Am J Infect Control. 2012;40:227–32.
13. Agency for Healthcare Research and Quality. Estimating the Additional Hospital Inpatient Cost and Mortality Associated with Selected Hospital-Acquired Conditions, 2017. Accessed 19 Jan 2024
14. Pitiriga, Kanellopoulos, P., Bakalis, I. et al. Central venous catheter-related bloodstream infection and colonization: the impact of insertion site and distribution of multidrug-resistant pathogens. Antimicrob Resist Infect Control 9, 189 (2020). https://doi.org/10.1186/s13756-020-00851-1