New Infographic Illustrates EVQ -218's Novel Approach to Combatting the World’s Worst Superbugs Without Triggering Antimicrobial Resistance
SALT LAKE CITY, Utah, (Sept. 26, 2024) — As the United Nations (UN) prepares to address the escalating threat of antimicrobial resistance, EVŌQ Nano is helping raise awareness through an educational infographic depicting a common trigger of antimicrobial resistance and highlighting EVQ-218’s novel mechanism of action.
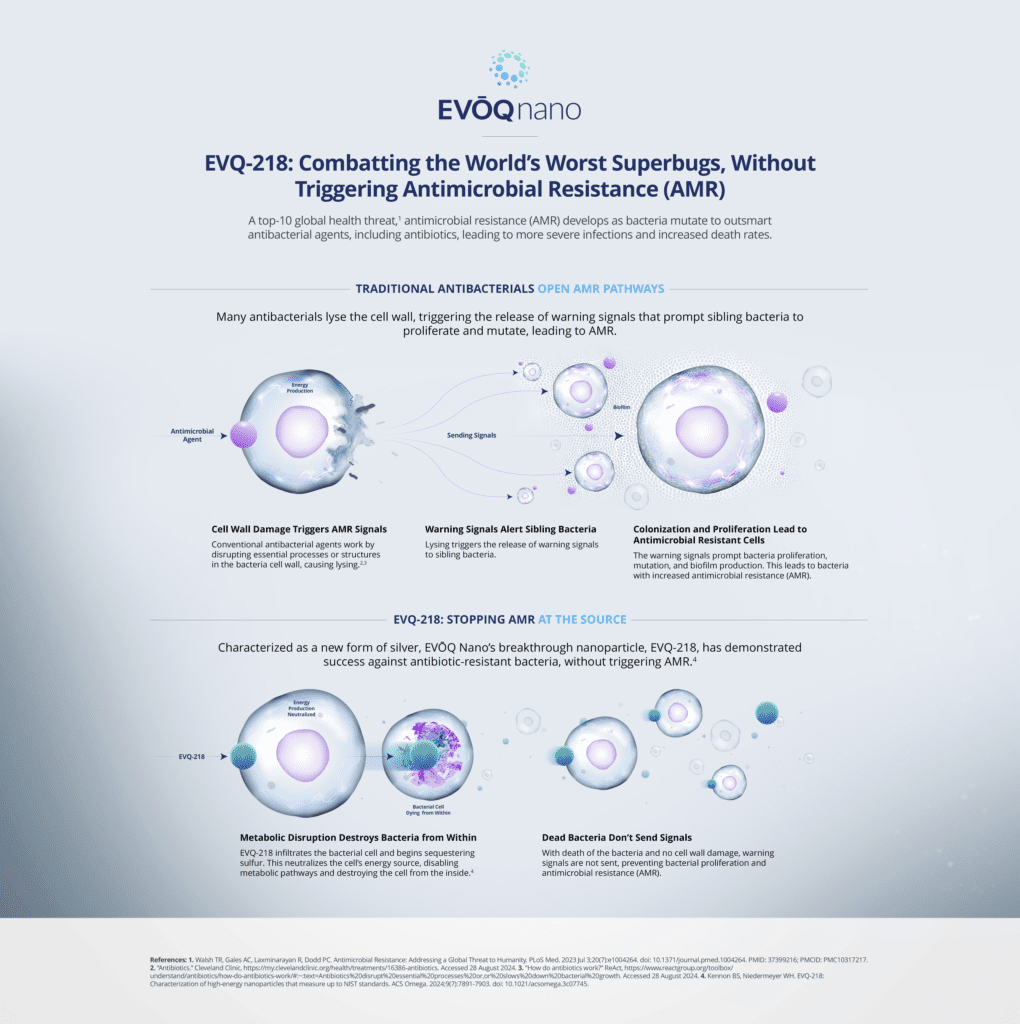
Antimicrobial resistance (AMR) — considered a top 10 global health threat1 — occurs when bacteria mutate to outsmart antibacterial agents, including antibiotics, leading to more severe infections and higher mortality rates. The UN General Assembly high-level meeting on AMR presents a critical opportunity for global stakeholders to address the growing threat.
Combatting Pulmonary Bacterial Infections
Bacteria’s ability to form biofilms is a key factor in their resistance to antibiotics. Biofilms are protective layers that shield bacteria from therapeutic interventions and the body’s immune system, especially in conditions like cystic fibrosis where infections become entrenched deep within the lungs.
Pulmonary therapeutics face hurdles in reaching the lower respiratory passages due to the lungs’ complex structure, mucus production, and natural clearance mechanism, which expels foreign particles. Recent preclinical trials of EVQ-218 show promise in addressing these challenges.
- In rodent studies, EVQ-218’s uniform, sub-10 nm nanoparticles effectively penetrated the distal regions of the lungs, overcoming typical delivery barriers. This deep lung penetration, combined with data showing efficacy against biofilm formation, positions EVQ-218 as a promising candidate for tackling difficult-to-treat infections.
- Serial passage assays, a critical test to assess the development of drug resistance over time, have also been conducted on EVQ-218. The results showed no development of resistance in 28-day and 35-day testing. Resistance to other antibiotics typically occurs in 4-5 days.2-6
“The rising challenge of antimicrobial resistance calls for innovative solutions,” said EVŌQ Nano CEO, Shaun Rothwell. “EVQ-218 represents a promising approach that could significantly impact this global health threat. We’re all in this fight together, and the more information we can share, the better equipped we’ll be to develop effective solutions.”
Advancing Toward Clinical Trials
EVŌQ Bio, a wholly-owned subsidiary of EVŌQ Nano, received two grants from the Cystic Fibrosis Foundation to develop an inhaled therapeutic using EVQ-218 to combat pulmonary bacterial infections associated with cystic fibrosis. The company recently completed a successful Pre-Investigational New Drug (pre-IND) meeting with the U.S. Food and Drug Administration and is confidently advancing toward Phase I clinical trials.
EVQ-218 has been characterized as a new form of silver by the Journal of the American Chemical Society, ACS Omega. Unlike conventional antibacterial agents that often trigger AMR by rupturing bacterial cell walls, EVQ-218 employs a novel mechanism of action that kills bacteria from the inside.7
- EVQ-218 infiltrates the bacterial cell and begins sequestering sulfur. This neutralizes the cell’s energy source, disabling metabolic pathways and destroying the cell from the inside.7
- With death of the bacteria and no cell wall damage, warning signals are not sent to sibling bacteria, preventing bacterial proliferation and AMR.7
In vitro studies found EVQ-218 demonstrated efficacy against a range of pathogens, including the top six antibiotic-resistant strains identified by The World Health Organization.8
- Acinetobacter baumannii, CR
- Pseudomonas aeruginosa, CR
- Escherichia coli, 3GCR
- Klebsiella spp., 3GCR
- Klebsiella spp., CR
- Enterobacter spp., SGCR
About EVŌQ Bio
EVŌQ Bio is a preclinical-stage nanotherapeutics company pioneering a nanomedicine platform with demonstrated antimicrobial efficacy. The company’s first therapeutic in development is for the treatment of pulmonary bacterial infections in patients with cystic fibrosis. The inhaled therapeutic utilizes EVQ-218, a groundbreaking nanoparticle that has demonstrated efficacy against a wide range of pathogens, including the top six antibiotic-resistant strains identified by the World Health Organization. Characterized as a new form of silver,7 EVQ-218 is the first and only non-ionic silver nanoparticle with effective antimicrobial action devoid of cytotoxicity. EVŌQ Bio is a wholly-owned subsidiary of EVŌQ Nano. To learn more, visit evoqbio.com.
About EVŌQ Nano
EVŌQ Nano is a nanoscience company that engineers novel nanoparticles for the life, materials, and textile science industries. The company’s multi-patented, high-volume laser nanofabrication process creates uniform, sub-10 nm nanoparticles with distinct surface chemistry. These properties represent a significant advancement in nanoscience with the potential for a wide range of applications. To learn more, visit evoqnano.com.
# # #
Media Contact
Capwell Communications
References
- “Antimicrobial Resistance.” World Health Organization, https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance#:~:text=Key%20facts,of%20modern%20medicine%20at%20risk. Accessed 4 Sept. 2024.
- Cakic M, Glisic S, Nikolic G. Synthesis, characterization and antimicrobial activity of dextran sulphate stabilized silver nanoparticles. J Mol Struct. 2016;1110:156–161. doi: 10.1016/j.molstruc.2016.01.040
- Yan X, He B, Liu L, Qu G, Shi J, Hu L, Jiang G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics. 2018;10:557–564. doi: 10.1039/C7MT00328E
- Gamboa SM, Rojas ER, Martínez VV, Vega-Baudrit J. Synthesis and characterization of silver nanoparticles and their application as an antibacterial agent. Int J Biosen Bioelectron. 2019;5(5):166-173. doi: 10.15406/ijbsbe.2019.05.00172
- Rajeshkumar S, Bharath LV. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem Biol Interact. 2017;273:219–227. doi: 10.1016/j.cbi.2017.06.019
- Mikhailova EO. Silver nanoparticles: mechanism of action and probable bio-Application. J Funct Biomater. 2020 Dec; 11(4): 84. Published online 2020 Nov 26. doi: 10.3390/jfb11040084
- Kennon BS, Niedermeyer WH. EVQ-218: Characterization of high-energy nanoparticles that measure up to NIST standards. ACS Omega. 2024;9(7):7891-7903. doi: 10.1021/acsomega.3c07745
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, including Tuberculosis. 2017;77. (Survey of world health experts)